Knockout of CTLA-4 can improve the antitumor efficacy of CAR-T cells
On September 29, 2023, Professor Carl June's team published a paper in the journal Immunity entitled: Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Their study showed that CRISPR-Cas9-mediated CTLA-4 loss improved CAR-T cell proliferation and antitumor efficacy.
Mechanistically, CTLA-4 deletion allows CD28 signaling and maintains CAR expression on T cell surfaces. In clinical studies, the loss of CTLA-4 saved T cell function in leukemia patients who had previously failed CAR-T therapy. Thus, selective loss of CTLA-4 reactivates T cells in patients with dysfunctional chronic lymphocytic leukemia (CLL), providing a novel strategy for increasing patient response to CAR-T cell therapy.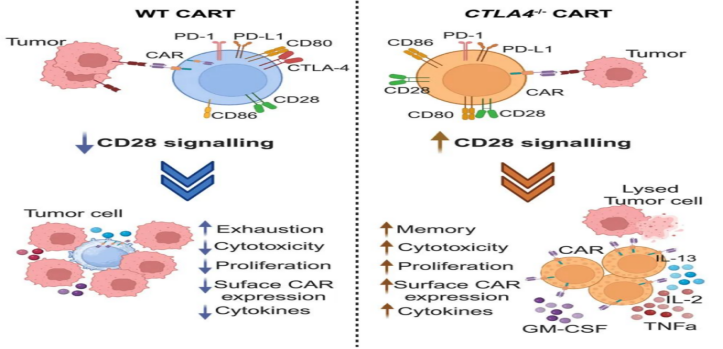
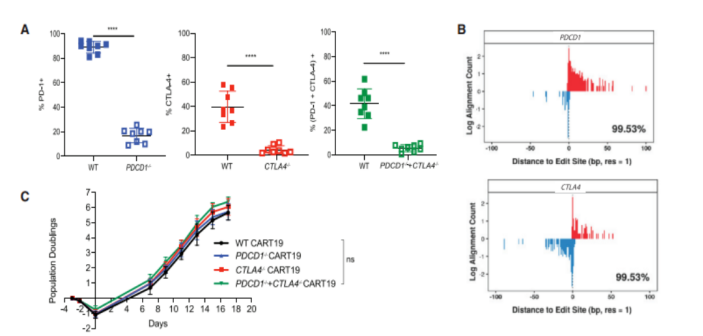
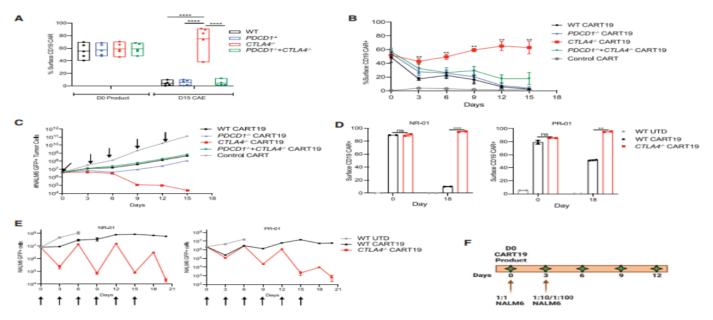
To uate whether CRISPR/ Cas9-mediated PD-1 and CTLA-4 checkpoint receptor deletion could improve the efficacy of CD19-targeting chimeric antigen receptor T cells (CART19), they prepared humanized CART19 cells for experiments. On the basis of ensuring effective knockout, they found that CART19 cells deficient in PD-1 and/or CTLA-4 exhibited similar proliferative capacity, memory phenotype, inhibitory receptor levels, and surface CAR expression. Similarly, WT and edited CART19 cells showed the same level of cytotoxic activity, degranulation ability, etc.
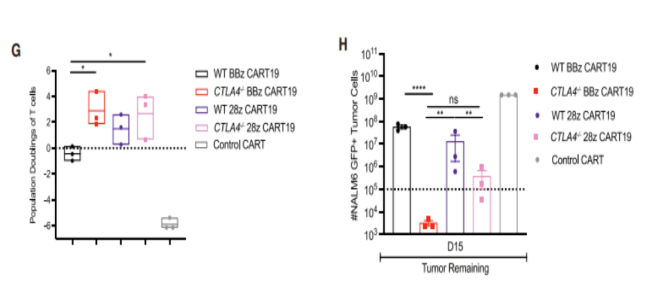
To determine whether loss of PD-1 and/or CTLA-4 could prevent or delay CAR-T dysfunction, they developed an in vitro stress test using chronic antigen exposure (CAE). We found that CTLA4-deficient CART19 cells exhibit double amplification and enhanced anti-tumor efficacy compared to PD-1-deficient CART19 cells and PD-1/CTLA-4 deficient CART19 cells.
In further stress tests, CTLA-4 deficient CART19 cells secreted increased levels of GM-CSF, IL-13, and CCL5 compared to both WT and PD-1-deficient CART19 cells and PD-1/CTLA-4 deficient CART19 cells. Under CAE stimulation, CTLA-4 deficient CART19 cells showed higher expansion and tumor clearance capacity than WT cells.
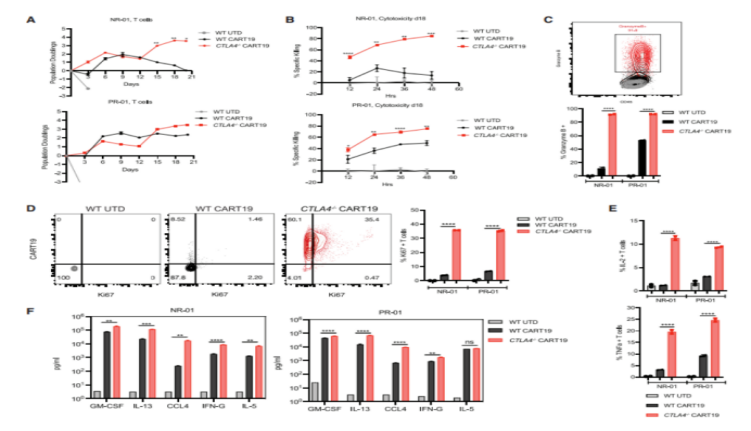
To determine whether CTLA-4 deletion activated dysfunctional T cells isolated from CLL patients, they obtained both WT and CTLA-4 deficient CART19 cells from patients with complete response (CR)-01, partial response (PR)-01, non-response (NR)-01, and NR-02. CTLA-4 deficient CART19 cells from CLL-unresponsive patients (NR-01) showed enhanced proliferative, persistent, and cytolysis activity at day 18 of stress compared to WT CART19. In addition, NR-01 and PR-01 CART19 cells with CTLA-4 deletion expressed higher levels of granzyme B and Ki67 levels, as well as elevated levels of cytokines IL-2 and TNF-α, confirming their excellent anti-tumor performance compared to WT NR-01 and PR-01 CART19 cells.
Loss of surface CAR expression in CAR-T cells leads to CAR-targeting dysfunction. CTLA-4 deficient CART19 cells showed higher anti-tumor efficacy in a CAR dysfunction model, which prompted them to examine relative levels of surface CAR expression in the WT and CART19 editing groups. CTLA-4 deficient CART19 cells maintained surface CAR expression in stress tests compared to both WT and PD-1-deficient cells and PD-1/CTLA-4 deficient CART19 cells. Similarly, WT CART19 cells from both NR and PRCLL patients showed a decreased percentage of surface CAR expression after repeated stimulation, while CTLA-4 missing CART cells maintained surface CAR expression and showed significantly improved cell-killing activity, thereby reducing tumor burden. They also verified these findings in a mouse model.
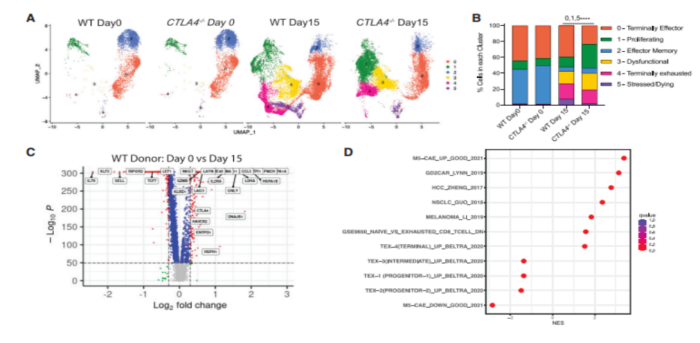
To investigate the molecular pathways driving CTLA-4 deficient CAR T cells to improve anti-tumor efficacy, they performed single-cell transcriptional sequencing (scRNA-seq) on WT and CTLA-4 deficient CAR T cells on days 0 and 15 after CAE stimulation. To gain insight into how the loss of CTLA-4 affects the widespread distribution of T cells, they mapped the relative number of cells in each subpopulation. Interestingly, on day 15, CTLA-4 deficient CART19 cells had a higher proportion of cells in the proliferating subpopulation and a lower proportion of cells in the end-effect subpopulation, stress subpopulation, and apoptosis subpopulation compared to WT CART19 cells. These data provide insight into how CTLA-4 deficient CAR T cells outperform WT CAR T cells. To identify dysfunction genes from in vitro stress dysfunction models, they determined differentially expressed genes on days 0 and 15 after CAE stimulation. They observed up-regulation of depletion genes such as CTLA-4, HAVCR2, LAG-3, KLRD1, IL-2RA, ENTPD1, GNLY, LAYN, CCL3, CCL4, TNFRSF18, and DUSP4.
To understand mechanistically how CTLA-4 deletion promotes improved anti-tumor efficacy of CAR-T cells in leukemia patients after stress testing, they identified and compared DEGs of WT and CTLA-4 deletion cells from ND and CLL CAR-T cells. There are many common up-regulated pathways in CTLA-4 deficient ND and CLL CAR-T cells, and glycolysis is the only one that is down-regulated. In ND and CLL CTLA-4 deficient CAR T cells after stress testing, pathways involved in cell cycle regulation were also present in the up-regulated pathway. This suggests that the absence of immune checkpoint CTLA-4 in CAR-T cells promotes the expansion of T cells.
They further examined the expression of downstream targets of CD28 in WT and CTLA-4 deficient ND and CLL CAR-T cells. CTLA-4 deficient CAR T cells showed up-regulation of GZMB, IL-2RA, and CDK6, which contribute to better proliferation and effector function. In addition, CD28 co-stimulation levels were enhanced in CTLA-4 deficient CART cells compared to WT CART19 cells. This was also replicated in xenografted mouse models, where increased expression of cell surface CD28 was detected in peripheral blood CAR T cells of CTLA-4 deficient mice compared to WT CLL CAR T cells. Based on these data, they propose that the loss of CTLA-4 in CAR-T cells leads to enhanced CD28 signaling, thereby maintaining co-stimulation of CAR-T cells and reducing CAR-T cell dysfunction, which together improve the effector function of CAR-T cells, increase their proliferation, and maintain CAR expression on the cell surface.
Mechanistically, CTLA-4 deletion allows CD28 signaling and maintains CAR expression on T cell surfaces. In clinical studies, the loss of CTLA-4 saved T cell function in leukemia patients who had previously failed CAR-T therapy. Thus, selective loss of CTLA-4 reactivates T cells in patients with dysfunctional chronic lymphocytic leukemia (CLL), providing a novel strategy for increasing patient response to CAR-T cell therapy.

To uate whether CRISPR/ Cas9-mediated PD-1 and CTLA-4 checkpoint receptor deletion could improve the efficacy of CD19-targeting chimeric antigen receptor T cells (CART19), they prepared humanized CART19 cells for experiments. On the basis of ensuring effective knockout, they found that CART19 cells deficient in PD-1 and/or CTLA-4 exhibited similar proliferative capacity, memory phenotype, inhibitory receptor levels, and surface CAR expression. Similarly, WT and edited CART19 cells showed the same level of cytotoxic activity, degranulation ability, etc.

To determine whether loss of PD-1 and/or CTLA-4 could prevent or delay CAR-T dysfunction, they developed an in vitro stress test using chronic antigen exposure (CAE). We found that CTLA4-deficient CART19 cells exhibit double amplification and enhanced anti-tumor efficacy compared to PD-1-deficient CART19 cells and PD-1/CTLA-4 deficient CART19 cells.
In further stress tests, CTLA-4 deficient CART19 cells secreted increased levels of GM-CSF, IL-13, and CCL5 compared to both WT and PD-1-deficient CART19 cells and PD-1/CTLA-4 deficient CART19 cells. Under CAE stimulation, CTLA-4 deficient CART19 cells showed higher expansion and tumor clearance capacity than WT cells.

To determine whether CTLA-4 deletion activated dysfunctional T cells isolated from CLL patients, they obtained both WT and CTLA-4 deficient CART19 cells from patients with complete response (CR)-01, partial response (PR)-01, non-response (NR)-01, and NR-02. CTLA-4 deficient CART19 cells from CLL-unresponsive patients (NR-01) showed enhanced proliferative, persistent, and cytolysis activity at day 18 of stress compared to WT CART19. In addition, NR-01 and PR-01 CART19 cells with CTLA-4 deletion expressed higher levels of granzyme B and Ki67 levels, as well as elevated levels of cytokines IL-2 and TNF-α, confirming their excellent anti-tumor performance compared to WT NR-01 and PR-01 CART19 cells.

Loss of surface CAR expression in CAR-T cells leads to CAR-targeting dysfunction. CTLA-4 deficient CART19 cells showed higher anti-tumor efficacy in a CAR dysfunction model, which prompted them to examine relative levels of surface CAR expression in the WT and CART19 editing groups. CTLA-4 deficient CART19 cells maintained surface CAR expression in stress tests compared to both WT and PD-1-deficient cells and PD-1/CTLA-4 deficient CART19 cells. Similarly, WT CART19 cells from both NR and PRCLL patients showed a decreased percentage of surface CAR expression after repeated stimulation, while CTLA-4 missing CART cells maintained surface CAR expression and showed significantly improved cell-killing activity, thereby reducing tumor burden. They also verified these findings in a mouse model.

To investigate the molecular pathways driving CTLA-4 deficient CAR T cells to improve anti-tumor efficacy, they performed single-cell transcriptional sequencing (scRNA-seq) on WT and CTLA-4 deficient CAR T cells on days 0 and 15 after CAE stimulation. To gain insight into how the loss of CTLA-4 affects the widespread distribution of T cells, they mapped the relative number of cells in each subpopulation. Interestingly, on day 15, CTLA-4 deficient CART19 cells had a higher proportion of cells in the proliferating subpopulation and a lower proportion of cells in the end-effect subpopulation, stress subpopulation, and apoptosis subpopulation compared to WT CART19 cells. These data provide insight into how CTLA-4 deficient CAR T cells outperform WT CAR T cells. To identify dysfunction genes from in vitro stress dysfunction models, they determined differentially expressed genes on days 0 and 15 after CAE stimulation. They observed up-regulation of depletion genes such as CTLA-4, HAVCR2, LAG-3, KLRD1, IL-2RA, ENTPD1, GNLY, LAYN, CCL3, CCL4, TNFRSF18, and DUSP4.
To understand mechanistically how CTLA-4 deletion promotes improved anti-tumor efficacy of CAR-T cells in leukemia patients after stress testing, they identified and compared DEGs of WT and CTLA-4 deletion cells from ND and CLL CAR-T cells. There are many common up-regulated pathways in CTLA-4 deficient ND and CLL CAR-T cells, and glycolysis is the only one that is down-regulated. In ND and CLL CTLA-4 deficient CAR T cells after stress testing, pathways involved in cell cycle regulation were also present in the up-regulated pathway. This suggests that the absence of immune checkpoint CTLA-4 in CAR-T cells promotes the expansion of T cells.
They further examined the expression of downstream targets of CD28 in WT and CTLA-4 deficient ND and CLL CAR-T cells. CTLA-4 deficient CAR T cells showed up-regulation of GZMB, IL-2RA, and CDK6, which contribute to better proliferation and effector function. In addition, CD28 co-stimulation levels were enhanced in CTLA-4 deficient CART cells compared to WT CART19 cells. This was also replicated in xenografted mouse models, where increased expression of cell surface CD28 was detected in peripheral blood CAR T cells of CTLA-4 deficient mice compared to WT CLL CAR T cells. Based on these data, they propose that the loss of CTLA-4 in CAR-T cells leads to enhanced CD28 signaling, thereby maintaining co-stimulation of CAR-T cells and reducing CAR-T cell dysfunction, which together improve the effector function of CAR-T cells, increase their proliferation, and maintain CAR expression on the cell surface.

Taken together, the study found and demonstrated that CTLA-4 deficient CART19 cells have superior anti-tumor efficacy under stress testing conditions, and more importantly, they confirmed the clinical relevance of these results, that the loss of CTLA-4 can enhance the fitness of human T cells. And was able to create an effective CART19 cell product from failed CLL patient T cells. In conclusion, the loss of CTLA-4 can activate patients' T cells, providing a new strategy for increasing long-lasting responses to CAR T cell therapy.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@duanglink.com
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@duanglink.com


