CRISPR screening reveals the role of BHLHE40 in mimicking CD8+ T cell exhaustion in vitro

Therefore, these systems are not ideal for high-throughput screening methods. E. John Wherry's team at the University of Pennsylvania has been working on T cell exhaustion-related research for many years. Today, the team published an article in Science Immunology titled In vitro modeling of CD8+ T cell exhaustion enables CRISPR screening to reveal a role for BHLHE40. They Through chronic stimulation in vitro, a model of CD8 T cell exhaustion was constructed, and based on the in vivo Tex model, the in vitro model was uated from the molecular phenotype, transcriptional level and epigenetic level, proving that it embodies a variety of in vivo Tex characteristics. , and then through CRISPR high-throughput sequencing of the in vitro model, the transcription factor BHLHE40 was screened out to regulate the T cell exhaustion process.
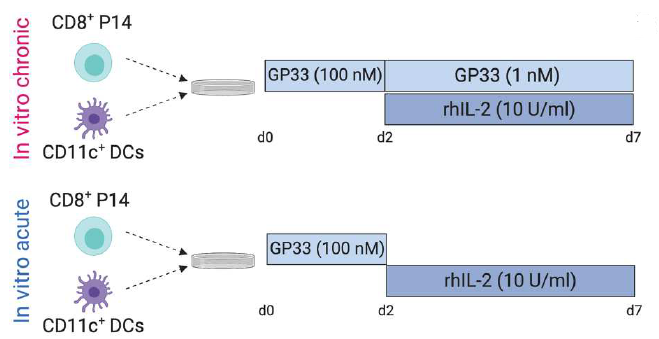
Subsequently, the in vitro depletion model was sequenced to probe its transcriptional and epigenetic features. RNA-seq results showed consistent chronic and acute model mapping with only 634 differential genes and 2844 differential genes at d7, suggesting similar initiation progression but differentiation at d7, where acute stimulation of P14 cells biased the Tmem phenotype and the Tex phenotype in the acute model. In gene enrichment analysis, genes upregulated by Tex in vivo were heavily enriched in P14 cells in the chronic model, and these genes were associated with negative regulation of T cell activation. In the ATAC-seq assay for epigenetic features, P14 cells in both models co-colocalized with Tex at d4, possibly related to the activation of relevant chromatin accessibility. Consistent with transcript levels, both showed a similar initial pathway as well as differentiation at d7.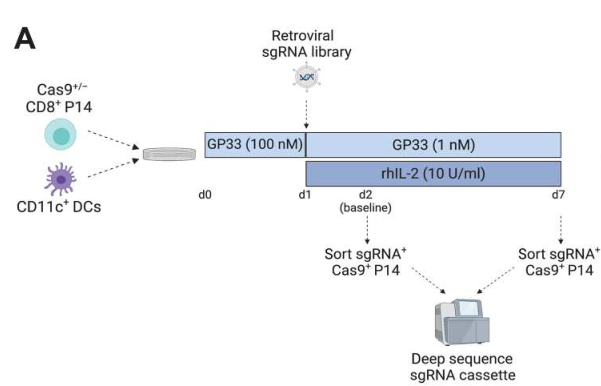
After proving that the chronic stimulation model in vitro can reflect the characteristics of Tex in vivo, the researchers used CRISPR screening in the hope of identifying relevant transcription factors that regulate the process of Tex. The results showed that BHLHE40 was highly expressed in the chronic model and did not appear in the acute model, suggesting that it may be closely related to T cell exhaustion.
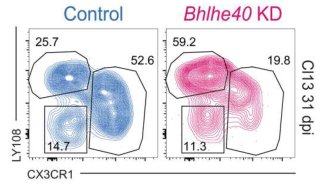
Researchers used shRNA to knock down BHLHE40 and found that PD-1, Tim3 and other IRs were down-regulated, suggesting that it may promote the expression of IRs. To test the function of BHLHE40 in regulating CD8 T cells in vivo, the researchers transferred BHLHE40 shRNA KD P14 cells into acutely and chronically infected mice and found that BHLHE40 knockdown cells in chronically infected mice had a stronger proliferation advantage. However, it was not shown in the acute model. Moreover, on d31, the expression of some IRs was reduced in BHLHE40 knockdown cells. At the same time, flow cytometry results showed that BHLHE40 knockdown promoted the differentiation of P14 cells from Tex metaphase cells to Tex progenitor cells.
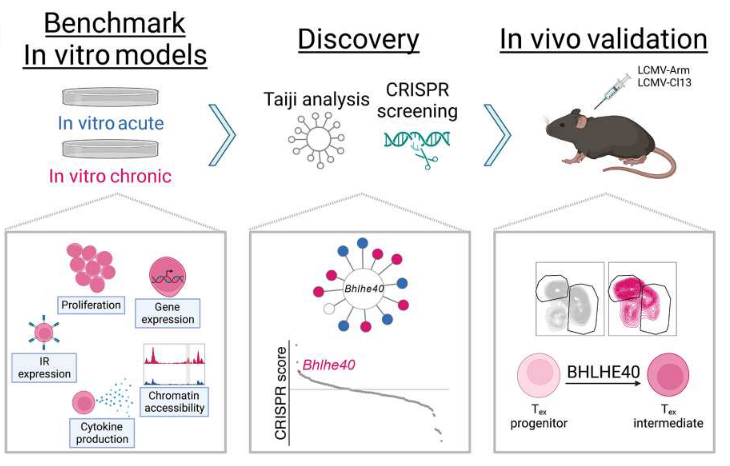
In summary, this study established an in vitro T cell exhaustion model, which can restore the characteristics of in vivo T cell exhaustion to a large extent. Although it cannot completely simulate the in vivo T cell exhaustion state, it can still be used for high-throughput sequencing, etc. study, and therefore found that the transcriptional regulator BHLHE40 plays an important role in the process of T cell exhaustion.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies and let more people know about the new development of biomedicine. The content of this article is only used for information exchange. This platform is neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information of this article shall not be used for diagnosis or treatment, can not substitute for professional medical opinion, the company website will not bear any responsibility. The final interpretation of the above statement belongs to the company website, this statement will apply to all the time shared articles, thank you for your cooperation! Copyright: The copyright of the article belongs to Shenzhen Cell Valley, and individuals are welcome to forward it to the circle of friends. Media or institutions, who will reprint it to other platforms in any form without authorization, will be regarded as infringement. If you need to reprint, please contact the email address: contact@duanglink.com
Next:Nothing



